hydrate formation chart
At γg 0704 the gas gravity chart shows the hydrate temperature to be 65F at 1050 psia. School University of Texas.

Hydrate Formation Curve An Overview Sciencedirect Topics
This chapter begins by presenting laboratory findings that synthetic anionic surfactants catalyze hydrate crystal nucleation greatly increasing rates and extents of hydrate formation.
. The hydrates with SH contain 34 water molecules per six cavities three cavities formed by12 pentagonal 512 two cavities formed by three square six pentagonal and three hexagonal faces 435663 and one large cavity formed by 12 pentagonal and eight hexagonal faces 5126825. Gas hydrate formation is a crystallization process whereby structured water must coincide with guest gases in a timely manner at appropriate temperatures and pressures. The chart method of Baillie and Wichert 1987 3.
Hydrate Formation Calculation 1000 psi 80 deg F 1000 psi 40 deg F 100 psi 80 deg. Typical guests molecules that form the type I hydrates are carbon dioxide and methane gas. Example Let say a natural gas has specific gravity 07 and operate at 50 o F.
Flow chart below shows the steps. There are three types of hydrates. Katz Abstract Charts for predicting the pressure to which natural gases may be expandedwithout hydrate formation.
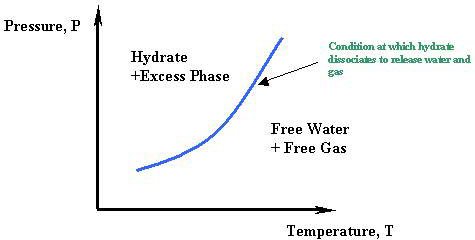
A general phase diagram for water hydrocarbon and solid hydrate is shown in Fig. A hydrate is any compound that has absorbed water molecules from its environment and included them in its structure. There are sixteen small cages and eight large cages in a unit cell.
Hydrate gaseous hydrocarbon excess liquid water Hydrate liquid hydrocarbon excess liquid water Ice gaseous hydrocarbon Liquid water gaseous hydrocarbon Liquid water liquid hydrocarbon. A graphical method has been developed that permits the estimation of the hydrate forming temperature at pressures in the range of 100 psia to 4000. Charts for predicting the pressure to which natural gases may be expandedwithout hydrate formation have been prepared for gases of even gravityPressure-temperature curves for hydrate formation were established for gaseshaving gravities from 06 to 10.
The hydrate formation acts as additional resistance. It is evident that the water stagnation points are most susceptible to hydrate formation. The cubic structure type II requires 136 molecules of water to form small and large cages.
The formation of hydrate has two major steps-nucleation and growth and is a time-dependent process. The knowledge of the dynamics of hydrate formation and accumulation of hydrate crystals is important in determining the parameters for mass production of gas hydrates and in understanding plug conditions in the gas pipeline and other equipment. Although computer programs employing equations of state are available to most engineers for the prediction of hydrate conditions field operations personnel will find useful the quick graphical approximation.
CSMHYD from the Colorado School of Mines release date Aug. Pages 77 Ratings 100 1 1 out of 1 people found this document helpful. The water molecules in inorganic hydrates are only loosely bonded to the compound and there is no chemical reaction.
Course Title TOPIC 7A. Seven methods for predicting hydrate formation will be examined in this paper. There are essentially five regions.
2 and the data in Table 5. Hydrate formation calculation 1000 psi 80 deg f 1000. What is hydrate formation pressure.
These curves and the thermal behavior of thegases during free and adiabatic expansion were used to prepare the. The region of high hydrate volume fraction in accumulated water is shown in figure 3. Deviations of the Calculated Hydrate Formation Temperatures from the Corresponding Experimental Data see a larger version Figure 2 shows the results of those correlations predicting the hydrate formation temperature for systems including ethylene glycol with less than 3 error.
Inorganic organic and gas or clathrate hydrates. Hydrate Formation in Multiphase Flow in Pipe By Seng Sook Harn 12842 Interim Report submitted in partial fulfillment of The requirements for the Bachelor of Engineering Hons Mechanical Engineering AUGUST 2013 Universiti Teknologi PETRONAS Bandar Seri Iskandar 31750 Tronoh Perak Darul Ridzuan CORE Metadata citation and similar papers at coreacuk Provided by. Prode Properties 119 includes a utility which allows to calculate the hydrate formation curve directly in Microsoft Excel the distribution files include a Excel page hydratexls from this page one can define composition including all common hydrate formers C1 Methane C2 Ethane C3 Propane C4 n Butane C4 i Butane N2 Nitrogen CO2 Carbon Dioxide.
The K-factor method of Mann et al. Prediction of Conditions of Hydrate Formation in Natural Gases Authors. Sum the amounts in steps 4 5 and 6 for the total methanol needed.
The original K-factor method hence forward simply referred to as the K-factor method 2. Start by calculating the gas gravity γg using Eq. The high hydrate volume fraction region is marked by the iso-surfaces of hydrate concentration which can be clearly distinguished from the water iso-surfaces.
Among these correlations the Bahadori-Activity and the Bahadori. Rate of Hydrate Formation. Steps to predict hydrate formation Pressure-temperature curve below is used to predict hydrate formation pressure or hydrate formation temperature for natural gas at certain specific gravity.
Step 1Calculate hydrate formation conditions using the gas gravity chart.

Typical Pressure Temperature P T Methane Hydrate Formation Diagram Or Download Scientific Diagram
Hydrate Formation Temperature Or Pressure Determination Oil And Gas Separator
Hydrate Formation Temperature Or Pressure Determination Oil And Gas Separator
Natural Gas Hydrates A Guide For Engineers
Sour Gas Hydrate Formation Phase Behavior Campbell Tip Of The Month

Hydrate Formation Temperature An Overview Sciencedirect Topics

Hydrate Formation Curve An Overview Sciencedirect Topics

Hydrate Natural Gas An Overview Sciencedirect Topics
The Equilibrium Conditions Of The Natural Gas Hydrate Formation Download Scientific Diagram
Sour Gas Hydrate Formation Phase Behavior Campbell Tip Of The Month
What Is The Impact Of Light Hydrocarbons On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month
The Hydrate Problem Png 520 Phase Behavior Of Natural Gas And Condensate Fluids

Hydrate Formation In Gas Systems Neutrium
Sour Gas Hydrate Formation Phase Behavior Campbell Tip Of The Month

Hydrate Formation Curve An Overview Sciencedirect Topics
Hydrate Formation Envelope Download Scientific Diagram
What Is The Impact Of Nitrogen On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month

Katz S Diagram For Estimation Of Hydrate Formation Conditions In Download Scientific Diagram
What Is The Impact Of Light Hydrocarbons On The Natural Gas Hydrate Formation Conditions Campbell Tip Of The Month
Comments
Post a Comment